The question is incomplete, here is the complete question:
Consider the reaction
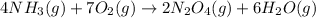
Initially,
![[NH_3(g)]=[O_2(g)]](https://img.qammunity.org/2021/formulas/chemistry/college/m0x04zq9h4vfpgssg0pa8lsqcfe65innkv.png) = 3.60 M; at equilibrium
= 3.60 M; at equilibrium
![[N_2O_4(g)]=0.60M](https://img.qammunity.org/2021/formulas/chemistry/college/bip0rwgep0yu6jjj1po95zr8lbmtqdug4f.png) . Calculate the equilibrium concentration for
. Calculate the equilibrium concentration for

Answer: The equilibrium concentration of ammonia is 2.8 M
Step-by-step explanation:
We are given:
Initial concentration of
![[NH_3(g)]](https://img.qammunity.org/2021/formulas/chemistry/college/ms0ai9w66bp5x0oec2zocikdi6d7p9lxli.png) = 3.60 M
= 3.60 M
Initial concentration of
![[O_2(g)]](https://img.qammunity.org/2021/formulas/chemistry/college/5yfw3515m0epb80r5qhaisuy71k4lcz0ng.png) = 3.60 M
= 3.60 M
For the given chemical equation:
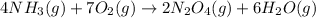
Initial: 3.60 3.60
At eqllm: 3.60-4x 3.60-7x 2x 6x
We are given:
Equilibrium concentration of
![[N_2O_4(g)]](https://img.qammunity.org/2021/formulas/chemistry/college/cso34rz2qy7j1qvcy6jrd5bn53pc5bvo12.png) = 0.60 M
= 0.60 M
Evaluating the value of 'x'
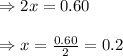
So, equilibrium concentration of
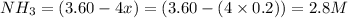
Hence, the equilibrium concentration of ammonia is 2.8 M