Step-by-step explanation:
Equation for the given reaction is as follows.
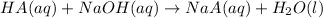
Therefore, moles of NaOH and HA are calculated as follows.
Moles of NaOH =
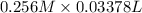
=
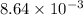
= 0.00864 mol
Moles of HA = 0.00864
Also, moles =
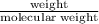
Molecular weight =
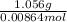
= 122.22 g/mol
Thus, we can conclude that molar mass of given unknown organic acid is 122.22 g/mol.