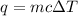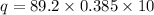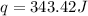Answer:
For Silver , The heat absorbed = 246.75 J
For Copper , The heat is = 343.42 J
Step-by-step explanation:
The change in temperature is calculated by:
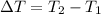
T2 = 25 C
T1 = 15 C
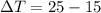
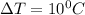
The energy in Joules can be calculated using :
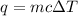
here , m = mass of the substance
c = the heat capacity
q = heat absorbed / released
We need to calculate the mass , In order to determine the value of "q".
Calculation for Silver :
The mass is calculated from the density of the element.
density of Silver = 10.5 g/ml (look at the table of density)
Volume of Cube = 10 cm^3 (given)
1 cm^3 = 1 mL
10 cm^3 = 10 mL
The mass can be calculated using the formula:
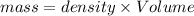
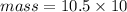
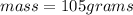
Insert the value of m , c, T in the equation.
for Silver the value of "c"= 0.235 J/gC (Look at the table)
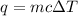
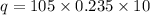
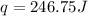
Calculation for Copper:
Again first calculate the mass of Copper.
Density of Copper = 8.92 g/ml
Volume = 10 mL
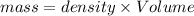
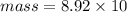
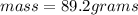
Insert the value of m , c, T in the equation.
for Silver the value of "c"= 0.385 J/gC (Look at the table)