The question is incomplete, here is the complete question:
If you performed a combustion reaction of 2-ethyl-1-methylpropene, what products would you expect to be present?
Answer: The products of the combustion of 2-ethyl-1-methylpropene are carbon dioxide and water.
Step-by-step explanation:
Combustion reaction is defined as the reaction in which a hydrocarbon reacts with oxygen gas to produce carbon dioxide gas and water molecule.
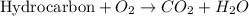
We are given a chemical compound, which is 2-ethyl-1-methylpropene. The chemical formula of this compound is
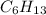
The chemical equation for the combustion of 2-ethyl-1-methylpropene follows:
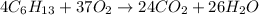
By Stoichiometry of the reaction:
4 moles of 2-ethyl-1-methylpropene reacts with 37 moles of oxygen gas to produce 24 moles of carbon dioxide and 26 moles of water
Hence, the products of the combustion of 2-ethyl-1-methylpropene are carbon dioxide and water.