The correct answer is option B - suboctet.
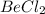 represents a scenario where the central atom, beryllium, does not achieve a full octet of electrons; it only has four valence electrons after forming two single bonds with chlorine atoms. This situation is an example of a suboctet, which occurs when a molecule has a central atom that is electron-deficient, having fewer electrons than needed for a noble gas configuration with an octet.
represents a scenario where the central atom, beryllium, does not achieve a full octet of electrons; it only has four valence electrons after forming two single bonds with chlorine atoms. This situation is an example of a suboctet, which occurs when a molecule has a central atom that is electron-deficient, having fewer electrons than needed for a noble gas configuration with an octet.
While compounds such as
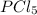 demonstrate an expanded octet, where the central atom has more than eight electrons in its valence shell,
demonstrate an expanded octet, where the central atom has more than eight electrons in its valence shell,
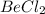 does not. Instead,
does not. Instead,
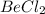 illustrates how some elements, particularly those in the second period such as beryllium, can form stable compounds with fewer than eight electrons around the central atom.
illustrates how some elements, particularly those in the second period such as beryllium, can form stable compounds with fewer than eight electrons around the central atom.