Answer: The percent composition by mass of hydrogen in given compound is 6.33 %
Step-by-step explanation:
We are given:
A chemical compound having chemical formula of
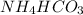
It is made up by the combination of 1 nitrogen atom, 5 hydrogen atoms, 1 carbon atom and 3 oxygen atoms
To calculate the percentage composition by mass of hydrogen in the compound, we use the equation:
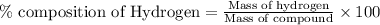
Mass of compound =
![[(1* 14)+(5* 1)+(1* 12)+(3* 16)]=79g/mol](https://img.qammunity.org/2021/formulas/chemistry/high-school/fi5uftki52wcokt9wlvrnm70w7ucuspjv1.png)
Mass of hydrogen =
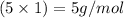
Putting values in above equation, we get:

Hence, the percent composition by mass of hydrogen in given compound is 6.33 %