Answer:
The concentration of the solution is 1.364 molar.
Step-by-step explanation:
Volume of perchloric acid = 29.1 mL
Mass of the solution = m
Density of the solution = 1.67 g/mL
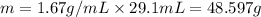
Percentage of perchloric acid in 48.597 solution :70.5 %
Mass of perchloric acid in 48.597 solution :
=
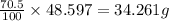
Moles of perchloric acid =
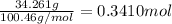
In 29.1 mL of solution water is added and volume was changed to 250 mL.
So, volume of the final solution = 250 mL = 0.250 L (1 mL = 0.001 L)
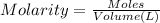
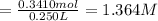
The concentration of the solution is 1.364 molar.