This is an incomplete question, here is a complete question.
What is the maximum number of moles of calcium sulfate that can be theoretically produced when 2.58 moles calcium hydroxide react with excess of sulfuric acid.
Answer : The maximum number of moles of calcium sulfate produced will be, 2.58 moles.
Explanation : Given,
Moles of calcium hydroxide = 2.58 moles
The balanced chemical reaction will be:
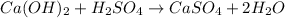
Now we have to determine the moles of calcium sulfate.
From the balanced chemical reaction we conclude that,
As, 1 mole of calcium hydroxide react to given 1 mole of calcium sulfate
So, 2.58 mole of calcium hydroxide react to given 2.58 mole of calcium sulfate
Thus, the maximum number of moles of calcium sulfate produced will be, 2.58 moles.