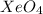The question is incomplete, here is the complete question:
Gaseous compound Q contains only xenon and oxygen. When a 0.100 g sample of Q is placed in a 50.0-mL steel vessel at 0°C, the pressure is 0.229 atm. What is the likely formula of the compound?
A. XeO
B.
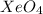
C.
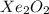
D.
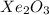
E.
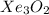
Answer: The chemical formula of the compound is
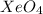
Step-by-step explanation:
To calculate the molecular mass of the compound, we use the equation given by ideal gas equation:
PV = nRT
Or,
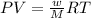
where,
P = Pressure of the gas = 0.229 atm
V = Volume of the gas = 50.0 mL = 0.050 L (Conversion factor: 1 L = 1000 mL)
w = Weight of the gas = 0.100 g
M = Molar mass of gas = ?
R = Gas constant =
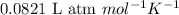
T = Temperature of the gas =
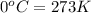
Putting value in above equation, we get:
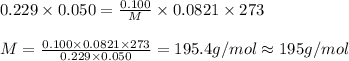
The compound having mass as 195 g/mol is
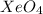
Hence, the chemical formula of the compound is