Answer:
The answer is C. Simple cubic, face-centered cubic, body-centered cubic
Step-by-step explanation:
Simple cubic: Containing one lattice point on each corner of the cube with each atom at the lattice point shared equally between the eight adjacent corners.
Body-centered cubic: having one lattice point in the center of the unit cell in addition to the eight corners. i.e
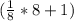
Face-centered cubic: having lattice points on the faces of the cube giving exactly one half contribution.