Answer : The correct answer is "No solution".
Explanation :
As we are given that 38.1 % w/w solution of heptane in chloroform. That means 38.1 gram of heptane present in 100 gram of solution.
Now we have to determine the mass of solution for 65.0 grams of hepatne.
As, 38.1 grams of heptane present in 100 grams of solution
So, 65.0 grams of heptane present in
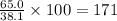 grams of solution
grams of solution
Since, there is only 20.0 grams of solution available. That means, there is not enough solution.
Thus, the correct answer is "No solution".