Answer: mole fraction of
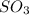 = 0.135
= 0.135
partial pressure of
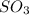 = 0.128 atm
= 0.128 atm
Step-by-step explanation:
The partial pressure of a gas is given by Raoult's law, which is:
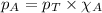 ......(1)
......(1)
where,
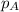 = partial pressure of substance A
= partial pressure of substance A
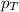 = total pressure
= total pressure
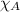 = mole fraction of substance A
= mole fraction of substance A
Mole fraction of a substance is given by:
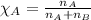
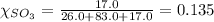
Putting the values in equation (1):
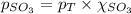
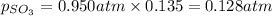
Thus the mole fraction of
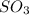 and its partial pressure are 0.135 and 0.128 atm.
and its partial pressure are 0.135 and 0.128 atm.