Answer : The ratio of the mass ratio of S to O in SO to the mass ratio of S to O in SO₂ is 2.
Explanation :
Law of multiple proportion : It states that when two elements can combine to form two or more different compounds then the mass of one element compared to fixed mass of the other will always be in a ratio of small whole numbers.
As we know that,
Mass of oxygen = 16 g
Mass of sulfur = 32 g
Mass of sulfur in SO = 32 g
Mass of oxygen in SO = 16 g
Mass ratio of Sulfur to oxygen in SO =
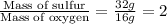 .....(1)
.....(1)
and,
Mass of sulfur in SO₂= 32 g
Mass of oxygen in SO₂ = (2 × 16)g = 32 g
Mass ratio of Sulfur to oxygen in SO₂ =
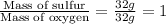 .....(1)
.....(1)
Ratio of S and O in SO = 2 : 1
Ratio of S and O in SO₂ = 1 : 1
Taking ratio of 1 and 2, we get:
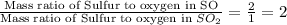
Hence, the ratio of the mass ratio of S to O in SO to the mass ratio of S to O in SO₂ is 2.