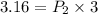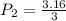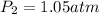Answer:
The new pressure is 1.0533 atm
Step-by-step explanation:
According to Boyle's Law : The Pressure of fixed amount of gas is inversely proportional to Volume at constant temperature.
PV = Constant
P1V1 = P2V2
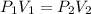 .....(1)
.....(1)
P1 = 3.16 atm
Accprding to question ,
V1 = V
V2 = 3 V
Insert the value of V1 , V2 and P1 in the equation(1)
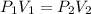
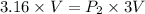
V and V cancel each other