To solve this problem we will apply the concepts related to kinetic energy and energy conservation. The kinetic energy will be expressed in terms of mass and speed, as well as load and voltage. From this last expression we will find the charges by electron and by Helium nucleus.
a ) Kinetic Energy is given as
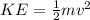
Replacing with our values we have that
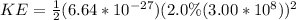
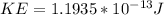
Therefore the kinetic energy of the helium nucleus is
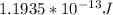
PART B) Now for calculate the electron volts we use the kinetic energy as a expression between the charge and the voltage, that is
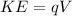
Here,
q = Charge of an electron
V = Voltage
Rearranging to find the potential we have,
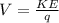
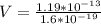
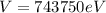
Therefore the kinetic energy in electron vols is
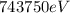
PART C) Applying the same relationship but now using the Helium core load, we will have to
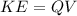
Here,
Q = Charge of a helium nucleus
V = Voltage
Rearranging to find the potential we have
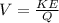
But we need to note that the charge is equal to the number of charge for the unit charge, then
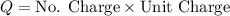
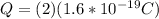
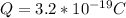
Now replacing we have that
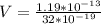
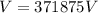
Therefore the voltage applied is 371875V