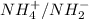Answer : The incorrect option is, (d)
Explanation :
According to the Bronsted Lowry concept, Bronsted Lowry-acid is a substance that donates one or more hydrogen ion in a reaction and Bronsted Lowry-base is a substance that accepts one or more hydrogen ion in a reaction.
Or we can say that, conjugate acid is proton donor and conjugate base is proton acceptor.
(a) The equilibrium reaction will be,
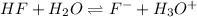
In this reaction,
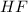 is an acid that donate a proton or hydrogen to
is an acid that donate a proton or hydrogen to
 base and it forms
base and it forms
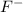 and
and
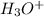 are conjugate base and acid respectively.
are conjugate base and acid respectively.
In this reaction,
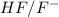 are act as a conjugate acid-base.
are act as a conjugate acid-base.
(b) The equilibrium reaction will be,
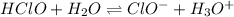
In this reaction,
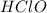 is an acid that donate a proton or hydrogen to
is an acid that donate a proton or hydrogen to
 base and it forms
base and it forms
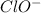 and
and
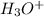 are conjugate base and acid respectively.
are conjugate base and acid respectively.
In this reaction,
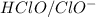 are act as a conjugate acid-base.
are act as a conjugate acid-base.
(c) The equilibrium reaction will be,
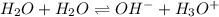
In this reaction,
 is an acid that donate a proton or hydrogen to
is an acid that donate a proton or hydrogen to
In this reaction,
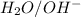 are act as a conjugate acid-base.
are act as a conjugate acid-base.
(d) The equilibrium reaction will be,
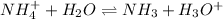
In this reaction,
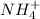 is an acid that donate a proton or hydrogen to
is an acid that donate a proton or hydrogen to
 base and it forms
base and it forms
 and
and
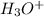 are conjugate base and acid respectively.
are conjugate base and acid respectively.
In this reaction,
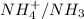 are act as a conjugate acid-base.
are act as a conjugate acid-base.
(e) The equilibrium reaction will be,
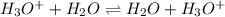
In this reaction,
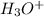 is an acid that donate a proton or hydrogen to
is an acid that donate a proton or hydrogen to
 base and it forms
base and it forms
In this reaction,
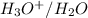 are act as a conjugate acid-base.
are act as a conjugate acid-base.
From this we conclude that that,
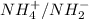 pairs of acids and conjugate bases is incorrectly labeled or incorrectly matched.
pairs of acids and conjugate bases is incorrectly labeled or incorrectly matched.
Hence, the incorrect option is, (d)