Answer:
The balance equation is
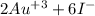 →
→
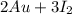
Step-by-step explanation:
first, we have to make sure that the atoms are balanced
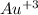 →
→
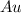
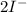 →
→
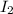
then we proceed to balance charges of each half-reaction
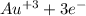 →
→
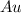
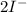 →
→
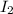
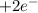
Now we multiply the half-reactions to match the number of electrons in each one
(
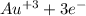 →
→
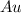 )x2
)x2
(
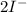 →
→
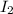
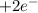 )x3
)x3
and now we do the sum of the half-reactions
2
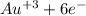 → 2
→ 2
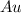
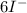 → 3
→ 3
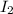
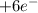
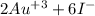 →
→
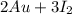
note: the only atom that needed to be balanced was I