Step-by-step explanation:
C:
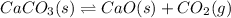 ΔH=+178 kJ/mol
ΔH=+178 kJ/mol
For an endothermic reaction, heat is getting absorbed during a chemical reaction and is written on the reactant side.
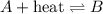
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle. This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
Treat heat as a reactant and on increasing a reactant at equilibrium, shifts the reaction in the forward direction.
Increase temperature → increase in heat → forward direction
Decrease temperature → decease in heat → backward direction
System C - Increase temperature : Reaction will move forward
System C - Decrease temperature : Reaction will move backward
D:
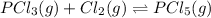 ΔH=−88 kJ/mol
ΔH=−88 kJ/mol
The total enthalpy of the reaction comes out to be negative .
The temperature of the surrounding will increase.
For an exothermic reaction, heat is released during a chemical reaction and is written on the product side.
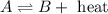
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle. This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
Treat heat as a product and on increasing a product at equilibrium, shifts the reaction in the backward direction.
Increase temperature → increase in heat → backward direction
Decrease temperature → decease in heat → forward direction
System D - Increase temperature : Reaction will move backward
System D - Decrease temperature : Reaction will move forward