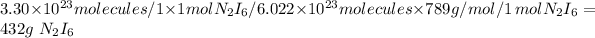432 grams of N₂I₆
Step-by-step explanation:
First we have to divide the given number of molecules (3.3\times10^2^8) by the Avogadro's number of molecules (6.022\times10^2^8), so that we will get the number of moles as 0.5.
Then the 0.5 moles of N₂I₆ is multiplied by the molar mass of N₂I₆ which is 789 g/mol, we will get the mass of N₂I₆ as 432 grams.
So the mass of the N₂I₆ obtained is 432 grams.