Answer:
0.0195 moles of N
Step-by-step explanation:
The molar mass of N is 14 g. I mole of an element is it's molar mass expressed in g.
so,
14 g N has 1 mole
0.273 g N will have
0.273 ÷ 14 = 0.0195 moles
or
n=
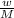 , where n= no of moles, w is mass of the atom or molecule and M is the molar mass of the atom or molecule.
, where n= no of moles, w is mass of the atom or molecule and M is the molar mass of the atom or molecule.
here, w=0.273g and M=14g
Substituting,
n=
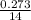 =).0195 moles
=).0195 moles