Answer:
The total number of charge pair without double counting is 6.
Step-by-step explanation:
Given that,
Number of proton = 2
Number of electron = 2
We need to calculate the total number of charges in Hydrogen atom
Using formula for number of charges
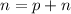
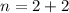
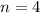
Number of charge in pair is
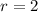
We need to calculate the total number of possible combination of pairs without double counting
Using formula of combination
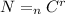
Where, n = number of charges
r = number of charges per pair
Put the value into the formula
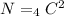
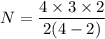
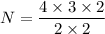
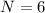
Hence, The total number of charge pair without double counting is 6.