Answer:
To draw the Lewis structure of one of the isomers of the C3H9N, propylamine is chosen, see attached drawing.
Step-by-step explanation:
- First, the electronic configuration of each atom involved is elaborated, in our case it would be nitrogen, hydrogen and carbon. This in order to determine the valence electrons.
N (Z=7)
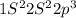 5 electrons
5 electrons
H (Z=1)
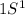 1 electron
1 electron
C (Z=6)
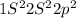 4 electrons
4 electrons
- The necessary electrons are sought for compliance with the octet rule. The carbons with their 4 links complies with the octet rule.
- To locate the non-bonding electrons, a difference is made between the total valence electrons and the linkers. The nitrogen atom has 6 assigned electrons and a non-binding pair.
- Finally the Lewis structure is made.