Answer:
Wavelength,
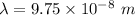
Step-by-step explanation:
We need to find the the wavelength of the light emitted by a hydrogen atom during a transition of its electron from the n = 4 to the n = 1 principal energy level. The energy is given by :
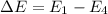
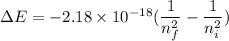
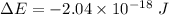
The energy of a photon is given by :
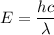
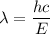
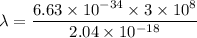
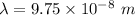
So, the wavelength of the light emitted by a hydrogen atom during a transition of its electron from the n = 4 to the n = 1 principal energy level is
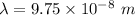 . Hence, this is the required solution.
. Hence, this is the required solution.