Answer : The ratio of
![([A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/zu05l2hw1fqjqd9x2ofm3ovlcqytwbmx51.png) at pH 5.75 is, 100
at pH 5.75 is, 100
Explanation : Given,
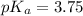
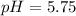
The equilibrium reaction of methanoic acid is,
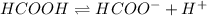
Using Henderson Hesselbach equation :
![pH=pK_a+\log ([Salt])/([Acid])](https://img.qammunity.org/2021/formulas/biology/college/z944fnahhldpjolfrvealc6q9baj5h69q3.png)
![pH=pK_a+\log ([HCOO^-])/([HCOOH])](https://img.qammunity.org/2021/formulas/chemistry/college/ug1uh4a832mobvzsnq9lj03au7chfpe30e.png)
or,
![pH=pK_a+\log ([A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/d36oy1cb96vgu3kbfgozmusy8vhw231m7h.png)
Now put all the given values in this expression, we get:
![5.75=3.75+\log ([A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/idzl8d03tattwqtzebyvin71a7s6mclagq.png)
![([A^-])/([HA])=100](https://img.qammunity.org/2021/formulas/chemistry/college/1juh88cg40s9mmip45lbmqp91cfcdk8l38.png)
Therefore, the ratio of
![([A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/zu05l2hw1fqjqd9x2ofm3ovlcqytwbmx51.png) at pH 5.75 is, 100
at pH 5.75 is, 100