Answer:
E = 4.75 x 10⁻¹⁶ J
Step-by-step explanation:
given,
wavelength of the x-ray , λ = 4.18 Å
Energy of photon = ?
we know
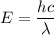
where h is the planks constant
c is the speed of light
h = 6.626 x 10⁻³⁴ m² kg / s
c = 3 x 10⁸ m/s
now,
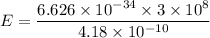
E = 4.75 x 10⁻¹⁶ J
hence, the energy of the photon is equal to E = 4.75 x 10⁻¹⁶ J