Answer:
NI3 has the highest molar mass = 394.707 grams
Step-by-step explanation:
1 mole of each substance contain mass which is equal to molar mass of the substance .
Molar mass is calculated in the same way the molecular mass is calculated.
The Molar mass id expressed in grams.
Molecular mass = Mass of the atom x the number of atoms
Atomic mass of H = 1.00 amu
O = 15.99 amu
P = 30.97 amu
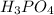 = 3(Mass of H) + 1(Mass of P) + 4(Mass of O)
= 3(Mass of H) + 1(Mass of P) + 4(Mass of O)
= 3(1)+ 1(30.97) + 4(15.99)
= 3 + 30.97 + 63.96
= 97.93 gram
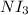 = 1 (mass of N) + 3(mass of I)
= 1 (mass of N) + 3(mass of I)
= 1(14.0067)+3(126.9)
= 394.7067 grams
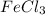 = 1(atomic mass of Fe) + 3(atomic mass of Cl)
= 1(atomic mass of Fe) + 3(atomic mass of Cl)
= 1(55.845)+3(35.45)
= 55.845 + 106.35
= 162.195 grams
KCl = 1(mass of K) + 1(mass of Cl)
= 1(39.09) + 35.45
= 74.54 gram
Out of these NI3 has the highest molar mass = 394.707 grams