Ferrosoferric oxide and hydrogen gas are the products of this reaction.
Step-by-step explanation:
Iron is the metallic element with the atomic number 26. It has a valency of both 2 and 3 which forms two types of oxide - ferrous oxide and ferric oxide.
Iron reacts with water with a metal and acid reaction, which forms both the iron oxide and evolves hydrogen gas. The hydrogen gas escapes the system and the mixture of iron oxide is left over in the system. The mixture is denoted by the formula
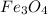 and is called ferrosoferric oxide.
and is called ferrosoferric oxide.
This reaction is reversible and is reversed if hydrogen gas is passed over heated ferric oxide in increased pressure.