Answer: C. a compound in which one or more carbon atoms have double or triple bonds.
Step-by-step explanation:
Saturated hydrocarbons are defined as the hydrocarbons in which single bond is present between C-C atoms. The general formula used for these hydrocarbons is
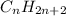 . Example:
. Example:
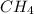
Unsaturated hydrocarbons are defined as the hydrocarbons which have double or triple covalent bonds present between carbon and carbon atoms. They are known as alkenes and alkynes respectively. The general formula used for these hydrocarbons is
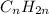 and
and
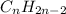 respectively. Example:
respectively. Example:
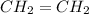
Both the hydrocarbons are not soluble in water as they are non polar in nature unlike water which is polar.
Cycloalkanes are also saturated hydrocarbons as single bond is present between C-C atoms.
Thus the correct statement is An unsaturated hydrocarbon is a compound in which one or more carbon atoms have double or triple bonds.