Answer:
1.30464 grams of glucose was present in 100.0 mL of final solution.
Step-by-step explanation:
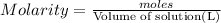
Moles of glucose =
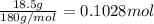
Volume of the solution = 100 mL = 0.1 L (1 mL = 0.001 L)
Molarity of the solution =
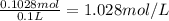
A 30.0 mL sample of above glucose solution was diluted to 0.500 L:
Molarity of the solution before dilution =
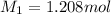
Volume of the solution taken =
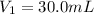
Molarity of the solution after dilution =

Volume of the solution after dilution=
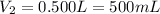

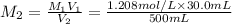
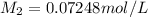
Mass glucose are in 100.0 mL of the 0.07248 mol/L glucose solution:
Volume of solution = 100.0 mL = 0.1 L
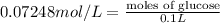
Moles of glucose =
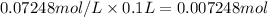
Mass of 0.007248 moles of glucose :
0.007248 mol × 180 g/mol = 1.30464 grams
1.30464 grams of glucose was present in 100.0 mL of final solution.