Answer:
The answer to your question is 15500 mg of MgSO₄ 7H₂O
Step-by-step explanation:
Data
Volume = 30 ml
Concentration = 0.3 M
Formula MgSO4∗7H2O
Process
1.- Calculate the number of moles needed
Molarity = moles / volume
Solve for moles
moles = Molarity x volume
Substitution
moles = 0.3 x 0.03 l
moles = 0.009 moles of MgSO₄
2.- Calculate the molecular mass of MgSO4∗7H2O
molecular mass = 24 + 32 + 64 + 14 + 112
= 246 g
3.- Get the proportion MgSO4 :7H2O, this proportion is 1 : 7
4.- Calculate the amount of MgSO4∗7H2O
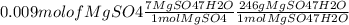
Simplification
15.5 g = 15500 mg