Answer:
The charge on the cation = 3
Step-by-step explanation:
According to Faraday's law of electrolysis , the mass of the substance deposited or liberated at any electrode is directly proportional to quantity of electricity passed.
Q= zIt
The charge can be calculated using :
Q = It
Here I = current in Ampere(A)
t = time in seconds(s)
Q = Charge in coulomb(C)
1 hours = 60 min and 1 minute = 60 second
1 hours = 60 x 60 second = 3600 sec
1 hrs = 3600 s
3.5 hrs = 3600 x 3.5 sec
= 12600 sec
I =0.15 A
t = 12600 sec
Q = It
Q = 0.15 x 12600 C
Now, the charge carried by 1 mole of electricity = 96500 C
Or,
96500 C is present in = 1 mole of electricity
1 C of charge is present in =
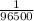
And 0.15 x 12600 C of electron contain:
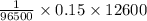 moles
moles
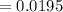 moles of electrons
moles of electrons
The Molar mass of the substance = 27 gram
The mass deposited = 0.176 gram
The moles deposited by the substance can be calculated by the following formula:
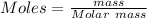
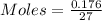
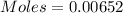 moles
moles
Now , the moles transferred (Magnitude of charge):
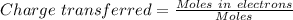
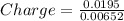
Charge transferred = 2.99(Nearly equal to 3 )
or
Charge transferred = 3