Answer:
(a)
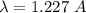
(b)
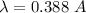
(c)
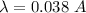
Step-by-step explanation:
Given that,
(a) An electron accelerated from rest through a potential difference of 100 V. The De Broglie wavelength in terms of potential difference is given by :
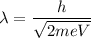
Where
m and e are the mass of and charge on an electron
On solving,
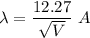
V = 100 V
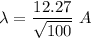
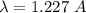
(b) V = 1 kV = 1000 V
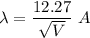
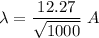
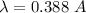
(c) If
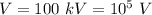
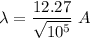
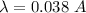
Hence, this is the required solution.