Answer:
The richer source of calcium is fluorite.
Step-by-step explanation:
Percentage of element in compound :
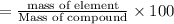
1. Dolomite is a carbonate of magnesium and calcium:
Given mass of dolomite = 7.81 g
Mass of calcium present in given mass of dolomite = 1.70 g
Percentage of calcium in Dolomite:
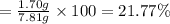
2. Fluorite is a mineral of calcium and fluorine:
Given mass of fluorite = 2.76 g
Mass of fluorine present in given mass of fluorite = 1.34 g
Percentage of fluorine in fluorite :
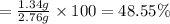
Percentage of calcium in fluorite = 100% - 48.55 % = 51.45%
Percentage of calcium in fluorite > Percentage of calcium in Dolomite
51.455 > 21.77%
So, the richer source of calcium is fluorite.