Answer :
(a) 1.000 g of compound containing carbon and hydrogen is, 0.922 g and 0.0769 g respectively.
(b) There is no other element present in the compound.
Explanation :
(a) Now we have to determine the masses of C and H in the sample.
The chemical equation for the combustion of hydrocarbon having carbon, hydrogen and oxygen follows:
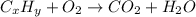
where, 'x' and 'y' are the subscripts of Carbon and hydrogen respectively.
We are given:
Mass of
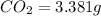
Mass of
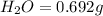
We know that:
Molar mass of carbon dioxide = 44 g/mol
Molar mass of water = 18 g/mol
For calculating the mass of carbon:
In 44 g of carbon dioxide, 12 g of carbon is contained.
So, in 3.381 g of carbon dioxide,
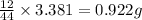 of carbon will be contained.
of carbon will be contained.
For calculating the mass of hydrogen:
In 18 g of water, 2 g of hydrogen is contained.
So, in 0.692 g of water,
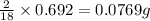 of hydrogen will be contained.
of hydrogen will be contained.
Thus, 1.000 g of compound containing carbon and hydrogen is, 0.922 g and 0.0769 g respectively.
(b) Now we have to determine the compound contain any other elements or not.
Mass carbon + Mass of hydrogen = 0.922 g + 0.0769 g = 0.999 g ≈ 1 g
This means that there is no other element present in the compound.