Answer:
The atomic mass of this isotope is 18.
Step-by-step explanation:
Given that,
Number of proton = 8
Number of neutron =10
Number of electron = 8
Atomic mass :
Atomic mass is the addition of number of neutron and number of proton or electron.
We need to calculate the atomic mass of this isotope
Using formula of atomic mass
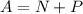
Where, N = number of neutron
P = number of proton
Put the value into the formula
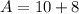
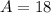
Hence, The atomic mass of this isotope is 18.