Answer:
The pH of the sweater containing Hydrogen ion concentration
![[H^(+)]=1* 10^(-8)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/3jofu9qwq1u1hzfwfrtonyz3x4ujm7ak9l.png) is
is
8
Step-by-step explanation:
pH = It is the negative logarithm of activity (concentration) of hydrogen ions.
pH = -log([H+])
Now, In the question the concentration of [H+] ions is :
![[H^(+)]=1* 10^(-8)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/3jofu9qwq1u1hzfwfrtonyz3x4ujm7ak9l.png)
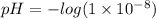
use the relation:
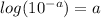
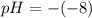
pH = 8
Note : 1 times 10 to the power of 8 must be" 1 times 10 to the power of -8"
If the concentration is
![[H^(+)]=1* 10^(8)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/n1wpxfyhjbuks1nyersywkhp2jbumrqpjh.png)
Then pH = -8 , which is not possible . So in that case the pH calculation is by other method