Answer:
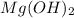 is the base in the reaction.
is the base in the reaction.
Step-by-step explanation:
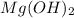 which is Magnesium hydroxide is a base because of the presence of Hydroxide ion. The pH of this magnesium hydroxide is 10 approximately. It is a strong electrolyte and has less solubility. Because of less solubility it becomes a weak base. This magnesium hydroxide is called as Milk of magnesia.
which is Magnesium hydroxide is a base because of the presence of Hydroxide ion. The pH of this magnesium hydroxide is 10 approximately. It is a strong electrolyte and has less solubility. Because of less solubility it becomes a weak base. This magnesium hydroxide is called as Milk of magnesia.
Acetic Acid + Magnesium Hydroxide = Magnesium Acetate + Water
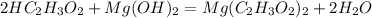
This is double displacement acid base reaction. Thus the reactant side must have an acid and an base. We could see that acetic acid is present hence Magnesium hydroxide must be the base involved in the reaction