Answer:
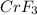 has greater impact on the freezing point depression of ice.
has greater impact on the freezing point depression of ice.
Step-by-step explanation:
Depression in freezing point is given by:
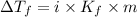
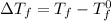 = Depression in freezing point
= Depression in freezing point
i= vant hoff factor
 = freezing point constant
= freezing point constant
m= molality
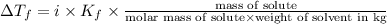
a) i = 4 for
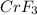 as it dissociates to give 4 ions in water.
as it dissociates to give 4 ions in water.
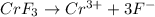
b) i = 3 for
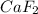 as it dissociates to give 3 ions in water.
as it dissociates to give 3 ions in water.
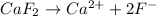
As the vant hoff factor is higher for
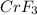 , it has greater impact on the freezing point depression of ice.
, it has greater impact on the freezing point depression of ice.