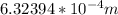Assuming the centripetal force is provided by the Coulomb attraction between the electron and the proton we have that,
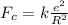
Here,
k = Coulomb's Constant
R = Distance
e = Electron charge
And by the centripetal force,
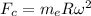
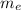 = Mass of electron
= Mass of electron
R = Radius
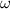 = Angular velocity
= Angular velocity
Equation both expression,
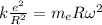
Replacing,
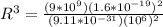
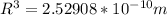
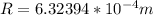
Therefore the radius of the electron orbit is