Answer:
0.370 micromoles of magnesium fluoride the chemist has added to the flask.
Step-by-step explanation:
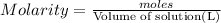
Moles of magnesium fluoride = n
Volume of the solution = 380.0 mL = 0.380 L (1 mL = 0.001 L)
Molarity of the solution =
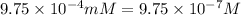
(1 mM = 0.001 M)
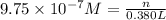
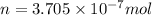
1 mole =
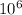 micro mole
micro mole
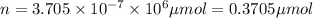
0.370 micromoles of magnesium fluoride the chemist has added to the flask.