Answer: The volume of isopropyl alcohol present is 254.4 mL and the volume of water present is 540.6 mL
Step-by-step explanation:
We are given:
32.0 % (v/v) alcohol solution
This means that 32 mL of isopropyl alcohol is present in 100 mL of solution
Volume of water = 100 - 32 = 68 mL
Volume of solution given = 795 mL
Applying unitary method:
In 100 mL of solution, the volume of isopropyl alcohol present is 32 mL
So, in 795 mL of solution, the volume of isopropyl alcohol present will be =
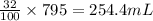
Volume of water in given amount of solution = 795 - 254.4 = 540.6 mL
Hence, the volume of isopropyl alcohol present is 254.4 mL and the volume of water present is 540.6 mL