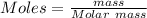Answer:
Hence the ions per mole are:
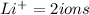
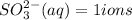
In one mole of Li2SO3 , the number of the atoms are
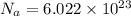
Step-by-step explanation:
The formula of lithium sulfite is Li2SO3.
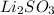
It contain Li+ and SO3(2-) ions.This can be represented by :
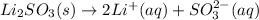
Hence one mole of Li2SO3 will give 2 Li+ ions and 1 SO3 (2-) ion.
Hence the ions per mole are:
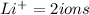
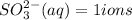
Number of atoms in lithium sulfite depends upon the mass of the Li2SO3 present .
Li2SO3 = 93.94 g/mole
This mass is equal to 1 mole of Li2SO3
Now 1 mole of any substance contain Na atoms . This is known as Avogadro Number.
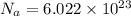
Hence , if 1 mole of Li2SO3 is present then it contains Na atoms
If other then 1 mole present then number of atoms are calculated by:
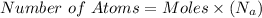
Here n = number of moles
if the mass of the compound is given then first calculate the number of moles.