Answer:
1.b. 2.5 mol
2.c.82.8%
3.d. synthesis
4.c . 2:2
5.d. 5 moles
6.c.5.02
7.d.3:1
8.d.2.68 moles
Step-by-step explanation:
1.
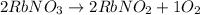
Here , 2 mole of RbNO3 will produce, 1 mole of O2
1 mole of RbNO3 will produce =
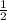 mole of O2
mole of O2
5 mole of RbNO3 will produce =

= 2.5 mole of O2
2 .
Molar mass of HCl = 1 + 35.45 = 36.45 gram
Molar mass of CO2 = 12 +32 = 44 gram
1 mole of the substance = molar mass
So , 1 mole of HCl = 36.45 gram
2 mole of HCl = 2 x 36.45 =72.9 gram
1 mole of CO2 = 44 gram
The given equation is :
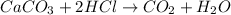
Here CaCO3 is the excess reagent and HCl is the limiting reagent
2 mole of HCl will produce = 1 mole of CO2
72.9 gram of HCl= 44 gram of CO2
so, 1 gram of HCl =
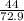 gram of CO2
gram of CO2
Here we need to calculate the mass of CO2 in 200 gram of HCl.
So, 200 gram of HCl =
 gram CO2
gram CO2
=120.71 gram CO2
this is the theoritical yield of CO2 = 120.71 gram
Experimental yield = 100 gram (given)
The percent yield is calculated by:
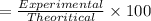
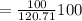
= 82.84%
3.Synthesis Reaction : Those reactions in which two or more substances combine to give a single product.
Here NH3 and HCl are combined and giving only one product = NH4Cl
4.
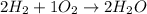
Hydrogen = H2
Water = H2O
Here Ratio of H2 to H2O is 2:2
5.The balanced equation is :
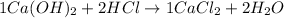
The number of atoms on left side should be equal to the right side:
Ca = 1
H = 4
O = 2
Cl = 2
Here, 2 moles of water(H2O) = 2 moles of HCl
So, 5 moles of water will give = 5 moles of HCl
6.
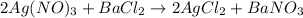
Molar mass of AgNO3 = 169.87 g/mol
1 mol of AgNO3 = 169.87 gram
Molar mass of AgCl = 143.32 g/mol
1 mole of AgCl = 143.32 grams
According to the given equation ,
2 mole of AgNO3 will give = 2 mole of AgCl
1 mole of AgNO3 = 1 mole of AgCl
169.87 gram of AgNO3 = 143.32 gram of AgCL
1 gram of AgNO3 =
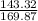
5.95 gram of AgNO3 =
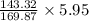
= 5.02 grams
7. The mole ratio of Hydrogen (H2) to Nitrogen (N2):
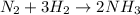
H2 = 3 mole
N2 = 1 mole
[H2]:[N2] = 3:1
8. The moles in a substance can be calculated by using :
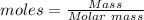
Mass of N2 = 75 grams (Given)
Molar mass = 28 gram
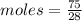
Moles = 2.678 moles