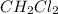Answer: The substance that produces fewest particles is
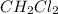
Step-by-step explanation:
Ionization reaction is defined as the reaction in which an ionic compound dissociates into its ions when dissolved in aqueous solution.
Covalent compounds do not dissociate into ions when dissolved in aqueous solution.
For the given options:
The chemical formula of sodium nitrate is
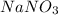
The ionization reaction for the given compound follows:

This produces in total of 2 ions.
- Option 2:
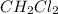
The given compound is a covalent compound and do not dissociate into its ions. It remains as such as a single unit.
- Option 3:
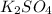
The chemical name for the given compound is potassium sulfate.
The ionization reaction for the given compound follows:
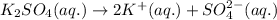
This produces in total of 3 ions.
- Option 4: Sodium phosphate
The chemical formula of sodium phosphate is
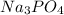
The ionization reaction for the given compound follows:
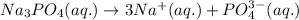
This produces in total of 4 ions.
Hence, the substance that produces fewest particles is