To solve this problem we will apply Henry's law. This law states that at a constant temperature, the amount of gas dissolved in a liquid is directly proportional to the partial pressure exerted by that gas on the liquid. Mathematically it is formulated as follows:
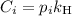
Here
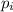 is the partial pressure of the gas.
is the partial pressure of the gas.
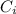 is the concentration of the gas (solubility).
is the concentration of the gas (solubility).
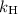 is Henry's constant, which depends on the nature of the gas, the temperature and the liquid.
is Henry's constant, which depends on the nature of the gas, the temperature and the liquid.
At the same time we know the the concentration can be defined as the relation between the number of moles and volume, then
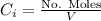
Then we have that the relation can be written now as,
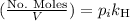
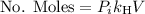
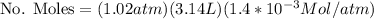
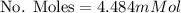
Now the fraction of Oxygen in that quantity is 0.21, then
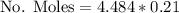
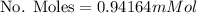
Mass of oxygen
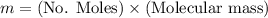
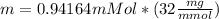
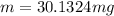
Therefore the mass of oxygen is 30.1324mg