Answer:
0.430
Step-by-step explanation:
The electronegative measures the power that the atom has to attract the electron to it. In a bond, if the elements have a huge difference of electronegativity, the electron will be lost by the less electronegative element and gained by the other, which will form ions. If the difference is not that large, they will share the electrons in covalent bonds.
But actually, the bonds are somewhere between ionic and covalent, and the ionic fraction of a bond can be calculated by the expression:
f =
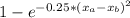
Where xa and xb are the electronegativities of the elements. So:
f =
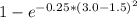
f = 0.430