Answer:
There are 5.98 electrons are missing from each ions.
Step-by-step explanation:
Given that,
The electric force between two identical charges is,
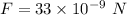
The distance between the charges,
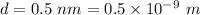
The electric force between charges is given by :
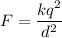
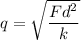
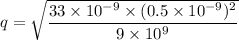

Let there are n number of electrons are missing from each ions. It is given by :
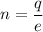
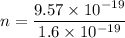
n = 5.98 electrons
So, there are 5.98 electrons are missing from each ions. Hence, this is the required solution.