Answer : The empirical and molecular formulas of acetic acid is,
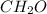 and
and
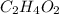 respectively.
respectively.
Solution :
If percentage are given then we are taking total mass is 100 grams.
So, the mass of each element is equal to the percentage given.
Mass of C = 40.00 g
Mass of H = 6.71 g
Mass of O = 100 - (40.00+6.71) = 53.29 g
Molar mass of C = 12 g/mole
Molar mass of H = 1 g/mole
Molar mass of O = 16 g/mole
Step 1 : convert given masses into moles.
Moles of C =
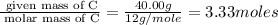
Moles of H =
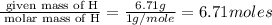
Moles of O =
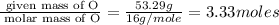
Step 2 : For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For C =
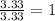
For H =
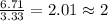
For O =
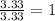
The ratio of C : H : O = 1 : 2 : 1
The mole ratio of the element is represented by subscripts in empirical formula.
The Empirical formula =
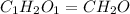
The empirical formula weight = 1(12) + 2(1) + 1(16) = 30 gram/eq
Now we have to calculate the molar mass of acetic acid.
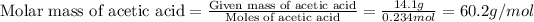
Now we have to calculate the molecular formula of the compound.
Formula used :
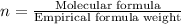
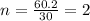
Molecular formula =
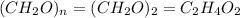
Therefore, the empirical and molecular formulas of acetic acid is,
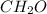 and
and
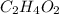 respectively.
respectively.