The question is incomplete, here is the complete question:
Calculate the volume in liters of a 0.13 M potassium dichromate solution that contains 200. g of potassium dichromate . Round your answer to 2 significant digits.
Answer: The volume of solution is 5.2 L
Step-by-step explanation:
To calculate the volume of solution, we use the equation used to calculate the molarity of solution:
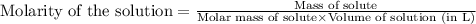
We are given:
Molarity of solution = 0.13 M
Given mass of potassium dichromate = 200. g
Molar mass of potassium dichromate = 294.15 g/mol
Putting values in above equation, we get:
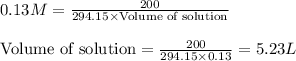
Hence, the volume of solution is 5.2 L