Answer:
10 moles
Step-by-step explanation:
The first thing to do is to write the balance chemical reaction
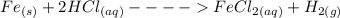
According to the balanced chemical equation, if the hydrochloric acid is in excess, one mol of iron forms one mole of iron (II) chloride, which is as many moles as iron. So 10 moles of iron will form 10 moles of iron (II) chloride.